How to Write Ionic Equations
For example sodium chloride melts at 801 C and boils at 1413 C. In reality you almost always start from the electron-half-equations and use them to build the.

How To Write The Net Ionic Equation For Hcl Zns H2s Zncl2 Chemical Equation How To Find Out Math
On this page well learn how to write symbol equations as well as how to calculate the number of moles of a substance.
. If an end of the tube is uncovered such that the air at the end of the tube can freely vibrate when the sound wave reaches it then the end is referred to as an open end. Whether to reference us in your work or not is a personal decision. Chemical equations are balanced for mass and.
C Combine these redox couples into two half-reactions. Ionic chemicals involve electrolytes which are substances that dissociate into ions when dissolved in polar solvents. If it is an academic paper you have to ensure it is permitted by your institution.
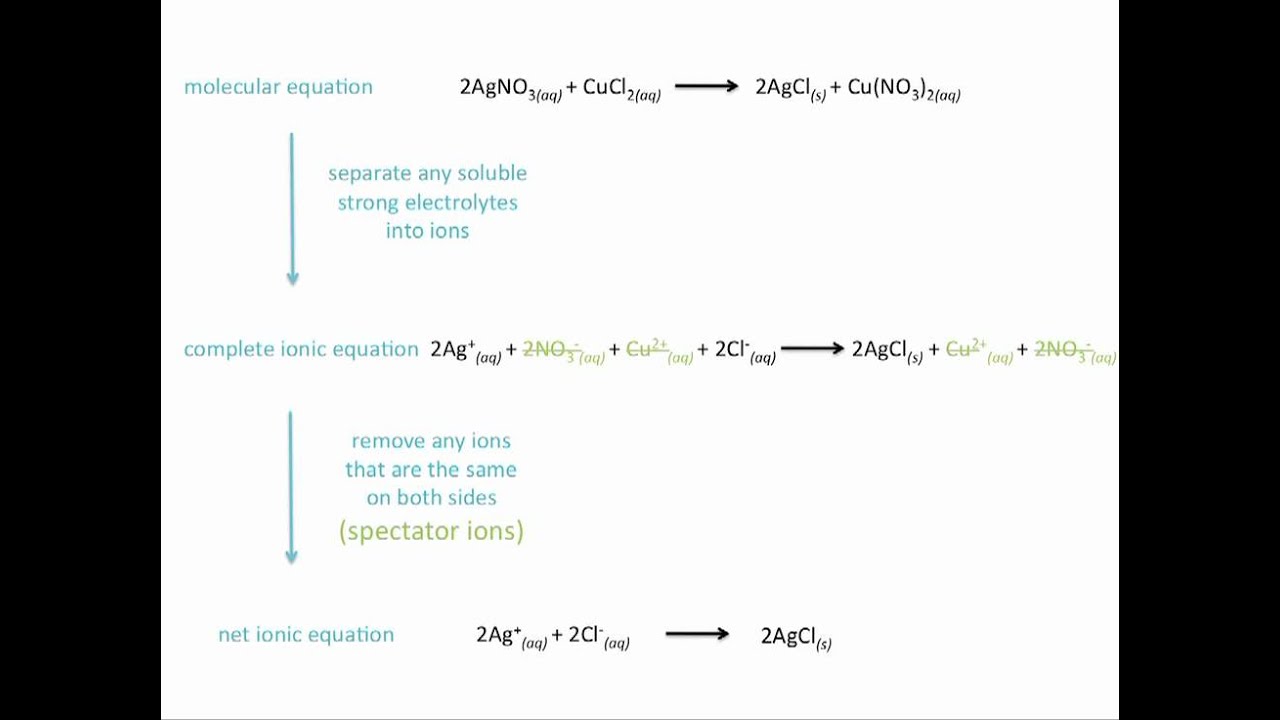
Translating chemical equations into ionic equations. There are different ways to write equations for chemical reactions. We do not ask clients to reference us in the papers we write for them.
Guidelines for balancing redox equations. Balancing for charge means the net charge is zero on both sides of the equation. Writing net ionic equations.
For best results a chemical balance calculator records the reaction in ion form. It makes it easy to see the active molecules in a reaction since they are the only ones present in the equation. All products and reactants must be known.
Chemical formulae equations and calculations. Electrolysis involves passing an electric current through either a molten salt or an ionic solution. They are often used to represent the displacement reactions that take place in aqueous mediums.
Write the imbalanced equation of the chemical reaction. The balancing equations calculator with steps divides the redox reaction into half of the reaction. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures.
These are half equations for. Increasing temperature concentration and surface area all increase the frequency of collisions. Ca 2 2e- Ca.
It is amazing how many marks are thrown away when writing about the collision theory. This means that you do not have to acknowledge us in. Write the molecular total ionic and net ionic equations illustrating the reaction.
In these reactions some ions participate in the reaction and some do not. The first step to writing a net ionic equation is balancing the chemical equation present. Explain how first and successive ionisation energies in Period 3 NaAr and in Group 2 BeBa give evidence for electron configuration in sub-shells and in shells.
As a comparison the molecular compound water melts at 0 C and boils at 100 C In solid form an ionic compound is not electrically conductive because its ions are unable to flow electricity. Then we rewrite the soluble ionic compounds as their dissociated ions. When we write papers for you we transfer all the ownership to you.
A chemical equation see an example below consists of a list of reactants the starting substances on the left-hand side an arrow symbol and a list of products substances formed in the chemical reaction on the right-hand sideEach substance is specified by its chemical formula optionally preceded by a number called stoichiometric coefficient. Question 15Write decomposition reactions for the following compounds aCaHCO 3 2 bAg 2 O cN 2 O 3 Question 16Write one equation each for. The state of matter aq stands for aqueous.
Balancing for mass produces the same numbers and kinds of atoms on both sides of the equation. It is not enough to say only there are more collisions as there is no reference to time. When we write papers for you we transfer all the ownership to you.
The quantities of substances produced or consumed by the electrolysis. This worksheet will help you practise writing ionic equations for neutralisation and precipitation reactions Where state symbols are not given youll need to use the solubility rules to determine whether a substance will ionise Write ionic equations for the following. The ions are forced to undergo either oxidation at the anode or reduction at the cathode.
Modification of work by vxlaFlickr. I bring thirty-two years of full-time classroom chemistry teaching experience and tens of thousands of hours of one-on-one chemistry tutoring across the globe to a seventeen year writing career that includes several best-selling international award-winning chemistry books and a burgeoning. A chemical equation describes what happens in a chemical reactionThe equation identifies the reactants starting materials and products resulting substances the formulas of the participants the phases of the participants solid liquid gas the direction of the chemical reaction and the amount of each substance.
Most electrolysis problems are really stoichiometry problems with the addition of an amount of electric current. Balanced chemical equations which indicate number and type of species. These are the spectator ions which we cancel out.
Example of Balanced Ionic Equation. The chemical equations in which electrolytes are represented in the form of dissociated ions are commonly referred to as ionic equations. O_2 Fe Fe_2O_3 Make a List.
The mole is a useful quantity. Write an unbalanced equation. Thats doing everything entirely the wrong way round.
They remain dissociated in solution. Whether to reference us in your work or not is a personal decision. As described above we use net ionic equations to emphasize the molecules that undergo a change in the reaction.
Ionic chemical equations are slightly different from that of a classic case of chemical equations. B Identify and write out all redox couples in reaction. An instrument consisting of a closed-end air column typically contains a metal tube in which one of the ends is covered and.
Many musical instruments consist of an air column enclosed inside of a hollow metal tube. Redox reactions are. A solution of AgNO 3 is mixed with a solution of K 2 S.
In the example above weve got at the electron-half-equations by starting from the ionic equation and extracting the individual half-reactions from it. More collisions per second is fine though. Write balanced formula unit total ionic and net ionic equations for the reaction Question 14.
When chemists measure out an amount of a substance they use an amount in moles. This is when you realise that some ions do not react. And net ionic equations which only deal.
Chemistry - how to write balanced ionic equations Molecular Complete Ionic and Net Ionic Equations How to write ionic and net ionic equations How to write a double replacement net ionic equation what are spectator ions precipitation reaction single displacement reaction with video lessons examples and step-by-step solutions. We do not ask clients to reference us in the papers we write for them. Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more.
A greater chance of. These electrolytes are split up and written as individual ions when written in ionic chemical equations. Balance the atoms in each half reaction a Balance all other atoms except H and O.
If it is an academic paper you have to ensure it is permitted by your institution. Modification of work by the Italian voiceFlickr. Im a true chemistry freelancer and Subject Matter Expert SME.
Modification of work. Working out electron-half-equations and using them to build ionic equations. Write equations for first and successive ionisation energies.
The ions that do not react are called spectator ions and are usually. This applies to both A-level and GCSE. Molecular equations which express compounds as molecules instead of component ions.
Write a balanced half equation for the formation of calcium from a calcium ion Ca 2. Some of the most common are unbalanced equations which indicate the species involved. We can write ionic equations by starting from their chemical equations.
When writing chemical equations we often use the symbol that an element is represented by on the Periodic table rather than writing out its full name. Separate the process into half reactions a Assign oxidation numbers for each atom. In aqueous solutions its common to balance chemical equations for both mass and charge.
This means that you do not have to acknowledge us in. HNO 3aq NaOH aq NaNO 3aq H 2 O l 2.

How To Write Ionic Equations Tutor Pace Science Chemistry Chemistry Class Chemistry Notes

Net Ionic Equation Worksheet And Answers Youtube Equations Chemistry Study Tips

How To Write A Net Ionic Equation In 2022 Ionic Redox Reactions Chemical Reactions

Ionic Equations Net Ionic Equations And Spectator Ions Chemistry Tutorial Chemistry Equations Ionic
Comments
Post a Comment